Knowledge
Polymerization and Degradation Monitored by EPR

In polymers, radical reactions are involved in the various processes. It is possible to investigate these phenomena by using EPR.
Polymerization and Degradation Monitored by EPR
The degradation of polymers due to light exposure leads to discoloration of the polymer and a decrease in the mechanical properties (elasticity, toughness, etc). To prevent this decomposition, light stabilizers are added to the polymer. By monitoring the EPR signals of these light stabilizer, their effectiveness can be evaluated using the EMXnano.
Introduction
In polymers, radical reactions are involved in the various processes such as polymerization, crosslinking and degradation. It is possible to investigate these phenomena by using EPR spectroscopy. These methods are used in both academic and industrial settings for research and quality control.
EPR is an important tool to study different polymerization reactions such as:
- Photo initiation and radical formation upon laser irradiation
- Polymerization reaction kinetics
- Cross-linking reactions
- Photo degradation analysis
- Inhibition effect of Hindered Amine Light Stabilizers (HALS) on UV degradation of polymers
Challenge
A wide range of reactions occur in polymers due to thermal and light activated mechanisms. These reactions are dominated by free radicals and influence both the creation and the destruction of the polymer.
Solution
Direct detection of the free radicals present in polymers during thermal or light treatment provide insight into the effective control of polymer formation and degradation.
Equipment
The EMXnano UV irradiation system and variable temperature system provide the ability to monitor free radicals during in-situ irradiation and thermalization of the sample of interest within the microwave cavity. The UV system features a light pipe to guide the UV light to the sample cavity interface thus assuring high light transmission efficiency and compliance with international safety standards. The variable temperature system uses an inert transfer gas to heat the sample to temperatures up to 500 K.
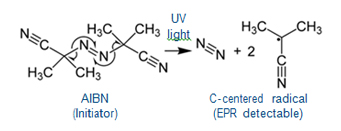
- The polymerization reaction of MMA (Methyl- Methacrylate) mixed with initiator AIBN (Azobisisobu-tyronitrile) at 50 °C during UV irradiation can be monitored using the EMXnano.
- Extracts from a 2D Field vs Time spectrum showing the evolution of the radicals formed during UV irradiation at 4 min (top) and 8 min (bottom).
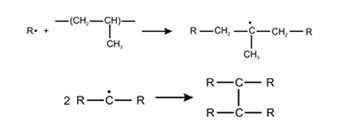
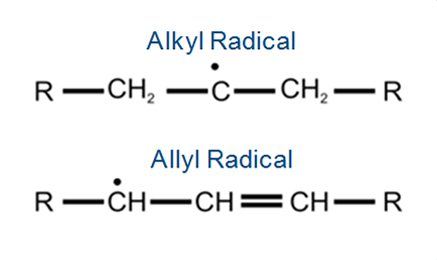
- Polyethylene (PE) in the presence of the cross-linking agent DCP (Dicumyl peroxide) forms two types of C-centered radicals while heating at 150 °C.
- At early times during the heating an Alkyl radical is formed (top) and after long term heat- ing an Allyl radical is formed (bottom).
- SpinCount uses the double integral value of the EPR spectrum to calculate the concentration of the PE radicals formed over time.
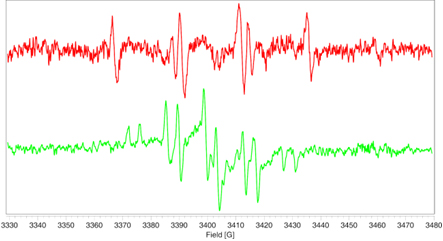
 PMMA without HALS
PMMA without HALS
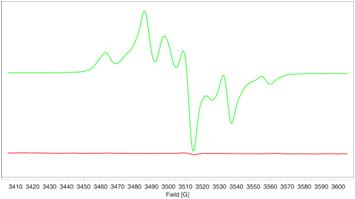
PMMA with HALS
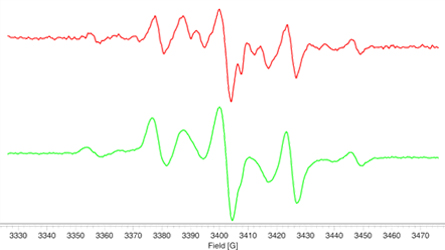
- The effectiveness of light stabilizers (HALS) for preventing polymer degradation by UV irradiation is easily evaluated with the EMXnano.
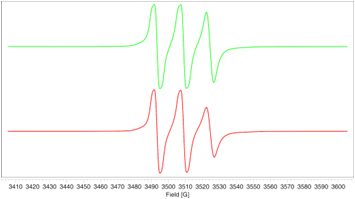
- The EPR signal generated in the polymer during UV irradiation (left) is completely suppressed after addition of the HALS stabilizer where only the HALS EPR spectrum is observed (right).
Key Features include:
- Easy-to-use software
- 2D EPR field sweep vs time experiments showing formation of polymer radicals
- SpinCount module to quantify the total number of spins and to determine the radical concentration
- The optional UV Irradiation System (100 W mercury lamp, 200 – 2000 nm wavelength range) provides the possibility for in-situ irradiation of the sample in the microwave cavity
Credit: Bruker
Contact us
388/5 Nuanchan Road, Nuanchan,
Buengkum, Bangkok 10230
0 2363 8585 (auto)
0 2363 8595
081 498 9939






